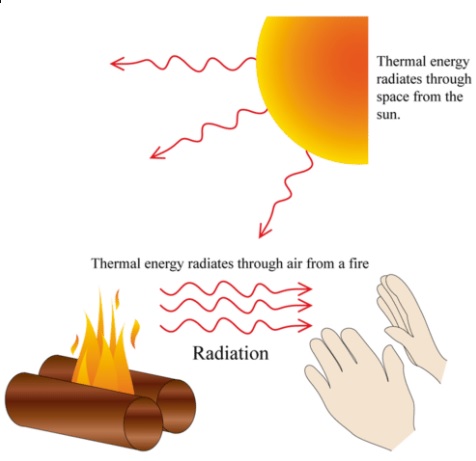
Consider a hot object that is suspended in an evacuated chamber whose walls are at room temperature. The hot object will eventually cool down and reach thermal equilibrium with its surroundings. This mechanism is radiation. Radiation transfer occurs in solids as well as liquids and gases. But heat transfer through an evacuated space can occur only by radiation. For example, the energy of the sun reaches the earth by radiation. It is interesting that radiation heat transfer can occur between two bodies separated by a medium colder than both bodies. The theoretical foundation of radiation was established in 1864 by physicist James Clerk Maxwell, Who postulated that accelerated charges or changing electric currents give rise to electric and magnetic fields. These rapidly moving fields are called electromagnetic waves or electromagnetic radiation.
Thermal Radiation
Thermal radiation is electromagnetic radiation generated by the thermal motion of particles in matter. All matter with a temperature greater than absolute zero emits thermal radiation. Particle motion results in charge-acceleration or dipole oscillation which produces electromagnetic radiation.
Infrared radiation emitted by animals (detectable with an infrared camera) and cosmic microwave background radiation are examples of thermal radiation.
If a radiation object meets the physical characteristics of a black body in thermodynamic equilibrium, the radiation is called blackbody radiation.[1] Planck’s law describes the spectrum of blackbody radiation, which depends solely on the object’s temperature. Wien’s displacement law determines the most likely frequency of the emitted radiation, and the Stefan–Boltzmann law gives the radiant intensity.
Thermal radiation is also one of the fundamental mechanisms of heat transfer. That is, everything around us such as walls, furniture, and our friends constantly emits (and absorbs) radiation. The type of electromagnetic radiation that is pertinent to heat transfer is the thermal radiation emitted as a result of energy transitions of molecules, atoms, and electrons of a substance. Thermal radiation is continuously emitted by all matter whose temperature is above absolute zero.
Thus, thermal radiation includes the entire visible and infrared (IR) radiation as well as a portion of the ultraviolet (UV) radiation.
There are 4 main properties that characterize thermal radiation:
- Thermal radiation emitted by a body at any temperature consists of a wide range of frequencies. The frequency distribution is given by Planck’s law of black-body radiation for an idealized emitter.
- The dominant frequency (or color) range of the emitted radiation shifts to higher frequencies as the temperature of the emitter increases.
- The total amount of radiation of all frequency increases steeply as the temperature rises; it grows, where the absolute temperature of the body.
- The rate of electromagnetic radiation emitted at a given frequency is proportional to the amount of absorption that it would experience by the source, a property known as reciprocity. Thus, a surface that absorbs more red lights thermally radiates more red lights.
Thermal radiation is one of the three principal mechanisms of heat transfer. It entails the emission of a spectrum of electromagnetic radiation due to an object’s temperature. Other mechanisms are convection and conduction.
Radiation heat transfer is characteristically different from the other two in that it does not require a medium and, in fact it reaches maximum efficiency in a vacuum. Electromagnetic radiation has some proper characteristics depending on the frequency and wavelengths of the radiation. The phenomenon of radiation is not yet fully understood.
We at KERONE have a team of experts to help you with your need for Thermal Radiation in various products range from our wide experience.