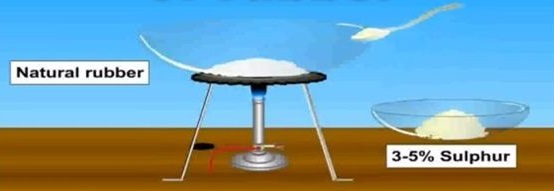
The process of heating natural rubber with sulphur is called vulcanization. Sulfur vulcanization may be a chemical process for changing natural rubber or related polymers into materials of variable hardness, elasticity, and mechanical durability by heating them with sulfur or sulfur-based curatives or accelerators. Sulfur forms cross-linking bridges between sections of polymer chains which have an effect on the mechanical and electronic properties. Many products are made with vulcanised rubber, as well as tires, shoe soles, hoses, and conveyor belts. The term vulcanization comes from Vulcan, the Roman god of fire.
The main polymers subjected to sulfur vulcanisation are polyisoprene (natural rubber, NR), polybutadiene rubber (BR) and styrene-butadiene rubber (SBR), all of that are made in unsaturated bonds. Many other specialty rubbers may also be vulcanised, like nitrile rubber (NBR), butyl rubber (IIR) and EPDM rubber. Vulcanisation, in common with the curing of other thermosetting polymers, is mostly irreversible. However, vital efforts have focused on developing ‘de-vulcanization’ processes for recycling of rubber waste.
The details of vulcanization remain murky as a result of the process converts mixtures of polymers to mixtures of insoluble derivatives. By design the reaction doesn’t proceed to completion because fully cross-linked polymer would be too rigid for applications. There has long been uncertainly on whether or not vulcanization proceeds during a radical or ionic manner.
Since the first 1900s, various chemical additives are developed to enhance the speed and efficiency of vulcanisation, as well as to control the character of the cross-linking. Once used together, this collection – the “cure package” – offers a rubber with specific properties.
The cure package consists of various reagents that modify the kinetics and chemistry of crosslinking. These include accelerants, activators, retarders and inhibitors. Note that these are simply the additives used for vulcanisation and that different compounds may also be added to the rubber, like fillers or polymer stabilizers.
- Accelerants
- Primary (fast-accelerants)
- Secondary (ultra-accelerants)
- Activators
- Retarders and inhibitors
The curing of rubber has been carried out since prehistoric times. The name of the primary major civilization in Guatemala and Mexico, the Olmec, means that ‘rubber people’ within the Aztec language. Ancient Mesoamericans, spanning from ancient Olmec’s to Aztecs, extracted latex from Castilla elastica, a sort of rubber tree within the area. The juice of a local vine, ipomoea Alba, was then mixed with this latex to create processed rubber as early as 1600 BCE. in the Western world, rubber remained a curiosity, although it was eventually used to manufacture waterproofed products, like Mackintosh rainwear, beginning within the early 1800s.
The discovery of the rubber-sulfur reaction revolutionized the use and applications of rubber, changing the face of the industrial world. Formerly, the only way to seal a small gap between moving machine parts was to use leather soaked in oil. This practice was acceptable only at moderate pressures, but above a certain point, machine designers were forced to compromise between the extra friction generated by tighter packing and greater leakage of steam. Vulcanized rubber solved this problem. It could be formed to precise shapes and dimensions, it accepted moderate to large deformations under load and recovered quickly to its original dimensions once the load is removed. These exceptional qualities, combined with good durability and lack of stickiness, were critical for an effective sealing material.
We at KERONE have a team of experts to help you with your need for Process Heating in various products range from our wide experience.