Sterilization indicates to any process that removes, kills, or deactivates all compositions of microorganisms such as fungi, bacteria, viruses, spores, unicellular eukaryotic organisms such as Plasmodium, etc. Sterilization can be attainted through diverse means, including heat, chemicals, irradiation, high pressure, and filtration. Sterilization is distinct from disinfection, sanitization, and pasteurization, in that those methods reduce rather than destroying all forms of life and biological agents present. After sterilization, an object is resorted to as being sterile or aseptic.
Chemicals are also accustomed for sterilization. Heating provides an infallible way to exterminate objects of all transmissible agents, but it is not always suitable if it will vandalize heat-sensitive materials such as biological materials, fiber optics, electronics, and many plastics. In these circumstances chemicals, either in a gaseous or liquid form can be used as sterilants. While the use of gas and liquid chemical sterilants avoids the problem of heat vandalize, users must ensure that the article to be sterilized is chemically compatible with the sterilant being used and that the sterilant is able to reach all surfaces that must be sterilized. In addition, the use of chemical sterilants poses new provocations for workplace protection, as the properties that make chemicals successful sterilants normally make them injurious to humans. The method for separating sterilant residue from the sterilized materials varies depending on the chemical and process that is used. The chemical method of sterilization can be classified as liquid and gaseous sterilization.
Chemicals Used In Sterilization.
- Ethylene oxide
- Nitrogen dioxide
- Ozone
- Glutaraldehyde and formaldehyde
- Hydrogen peroxide
- Peracetic acid
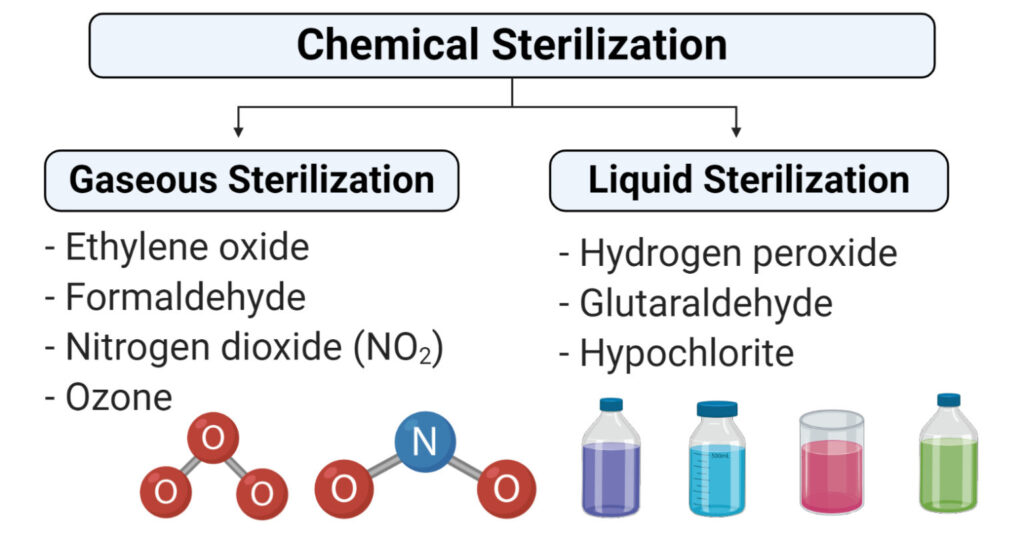
Gaseous Sterilization
- Gaseous sterilization engages the process of exposing equipment or devices to non- identical gases in a closed heated or pressurized chamber.
- Gaseous sterilization is a more successful technique as gases can pass between tiny orifices and give more successful outcome.
- Besides, gases are often used along with heat treatment which also smoothens the functioning of the gases.
- However, there is a matter of release of some toxic gases throughout the process which needs to be eliminated at regular intervals from the system.
- The mechanism of action is non-identical for divergent types of gases.
Liquid Sterilization
- Liquid sterilization is the process of sterilization which involves the submerging of equipment in the liquid sterilant to eliminate all feasible microorganisms and their spores.
- Although liquid sterilization is not as effective as gaseous sterilization, it is appropriate in conditions where a low level of defilement is present.
Psychological Sterilization
It can be strenuous to measure the psychological outcomes of sterilization, as definite psychological phenomenon may be more common in those who finally decide to take part in sterilization. The relationships between psychological problems and sterilization may be due more to correlation preferably than causation. That being said, there are several trends surrounding the psychological health of those who have received sterilizations. A 1996 Chinese study found that “risk for depression was 2.34 times greater after tubal ligation, and 3.97 times greater after vasectomy. If an individual goes into the course of action after being coerced or with a lack of understanding of the plan of action and its consequences, he or she is more likely to suffer negative psychological consequences afterwards. However, most people in the United States who are sterilized keep the same level of psychological health as they did preceding to the course of action. Because sterilization is a largely irreversible procedure, post-sterilization regret is a major psychological effect.
We at KERONE have a team of experts to help you with your need for Sterilization Equipment’s from our wide experience. For any query write us at [email protected] or visit www.kerone.com.
