Most materials are mixtures, and their behavior is usually very different from that of their parts in isolation. Amorphous metal mixtures, so-called metallic glasses, promise materials with extreme toughness and strength. However, spontaneous separation of the constituents destroys the benefits of the mixture, and results in crystallization in amorphous materials. This separation is vital not solely in synthetic glasses but also in volcanoes, where crystallization of the magma has profound consequences for its flow properties and might result in catastrophic volcanic explosions. Here, we reveal a universal mechanism for crystallization in mixtures, and we show that when the individual components without delay crystallize, which is nearly always the case, the mixture is vulnerable to crystallization.
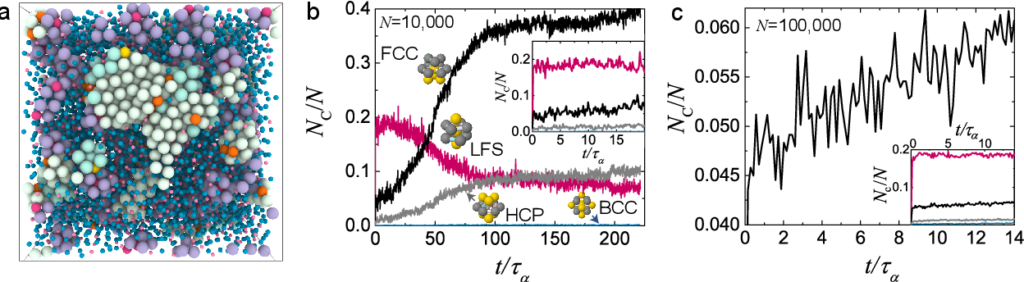
Crystallization in super cooled liquids has profound implications in fields as diverse as the development of amorphous materials, magma flows in volcanos, and aqueous solutions of ions. Materials in question include metallic, inorganic, and chalcogenide glass formers, where mixtures of a number of different constituents have the effect of suppressing or controlling crystallization [4]. Alas, this tendency to crystallize places stringent limits on the size of the pieces of amorphous material that can be formed: large pieces are more likely to undergo crystal nucleation. This “Achilles heel” of glass formation thus limits the exploitation of metallic glasses, for example, whose superior mechanical properties otherwise hold great promise.
It is clear that any liquid cooled below its freezing point must, for a sufficiently massive system, nucleate. However, the practical limits of cooling rate versus system size needed for vitrification aren’t known generally. In further to those practical issues, crystallization is one solution to the Kauzmann paradox of vanishing configurational entropy upon which a number of theories of the glass transition rest crystallization avoids the requirement to invoke any particular theoretical description of divergent viciousness in amorphous materials.
It is known empirically that increasing the amount of constituent species and introducing a size inequality among these parts, together with a negative heat of blending, tends to suppress nucleation—this has been the guiding principle in the development of bulk metallic glasses. However, despite recent innovative approaches using model systems and novel sampling techniques, there’s still a scarcity of fundamental understanding of the mechanisms by which glass-forming mixtures crystallize.
We at KERONE have a team of experts to help you with your need for crystalisation systems in various products range from our wide experience.
