Spray drying is one among the most remarkable technologies presently to be applied to pharmaceuticals. It’s a continuous process that converts, in a single step, a liquid feed into a powder and is an ideal method once precise attributes like particle size, morphology and stability are needed. This review describes the technology, current and future applications and how the current level of understanding and modeling tools enable a process development stage that’s both lean and risk-free.
Spray drying is a drying method that was firstly described more than 140 years ago as an improvement in drying and concentrating liquids1. But it was not until the beginning of the 20th century that the level of sophistication and knowledge of the process allowed its industrial use. The production of milk powder was the first commercial application and still remains one of the most important uses of the technology.
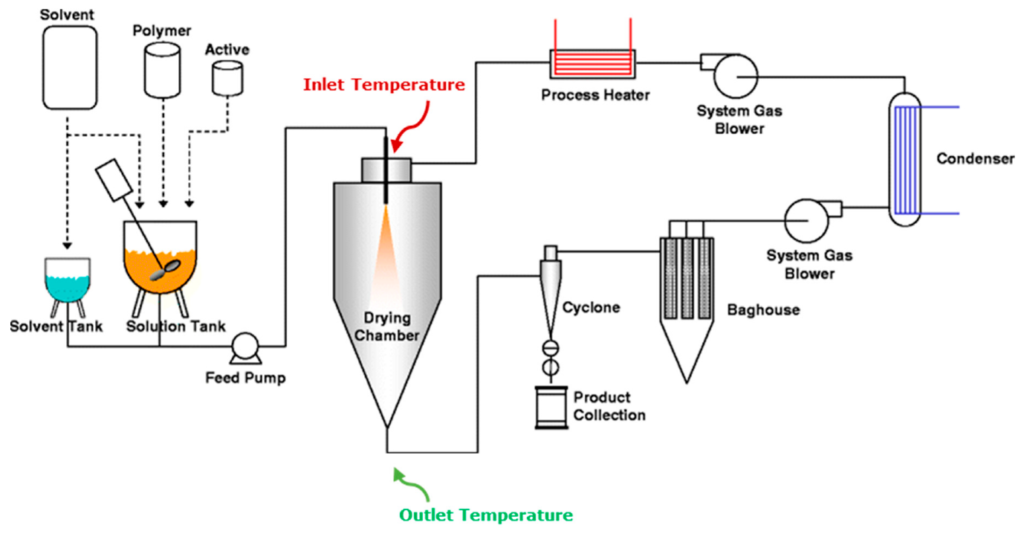
Spray drying involves the atomization of a liquid feed into very small droplets within a hot drying gas leading to flash drying of the droplets into solid particles. The particles are then separated from the drying gas, using a cyclone and/or a filter bag, as a final spray dried product. The feed can be a solution, a suspension or an emulsion and the resulting product can be classified as a powder, granules or agglomerates.

In a single continuous step, spray drying therefore converts a liquid feedstock into a powder with well-defined properties. Properties such as level of moisture or residual solvent in the powder, particle morphology or size and powder density can be manipulated to a great extent to target levels. The remarkable flexibility in tailoring the properties of the final powder, the gentleness of the process and its economics when compared with competing technologies such as freeze drying led to its proliferation in multiple industrial applications including cosmetics, fine chemicals, detergents, polymers, excipients and pharmaceuticals.
Spray dryers differ in many different ways: for example, whether or not the drying gas recirculates (close vs. open loop systems), the type of drying gas utilized (air, nitrogen or argon), the type of atomiser or nozzle (pressure, two-fluid, ultrasonic or rotary), the powder recovery system used (often through a cyclone and/or a filter bag), the degree of finishing (namely the level of polish of the internal surfaces), the existence or not of clean-in-place (CIP) or sterilization-in-place systems as well as the level of instrumentation and automation.
Pharmaceutical spray dryers usually combine the highest degree of finishing and the most sophisticated control systems with the simplest hardware designs that give for easier cleanup. In several cases nitrogen is the preferred drying gas to air, not solely because it permits the safe drying of organic solvents however also to reduce oxidation in the product. Pressure and two-fluid nozzles are largely utilized in pilot and enormous scale equipment and therefore their choice often depends on the kind of feed and the target particle size of the final product. Rotary nozzles are less often used due to poor clean ability. Very interesting is the use of ultrasonic nozzles in little scale equipment since they permit for the formation of enormous droplets which very closely match those of larger equipment.
For amorphous solid dispersions, close system spray dryers using nitrogen as the drying gas are typically used because of the need to handle organic solvents.
Pressure nozzles are also favored because they result in powders with better flow characteristics (as a result of the narrower particle size distribution), which simplifies the downstream processes of tableting or capsule filling.
